Introduction
Myelodysplastic syndrome-refractory cytopenia of childhood (MDS-RCC) and severe aplastic anemia (SAA) are rare acquired pediatric bone marrow failure (aBMF) diseases with shared clinical features but differing pathophysiology. Hematopoietic cell transplantation (HCT) is an effective curative therapy for both conditions. While HCT has shown excellent outcomes for SAA, reports on the outcome of HCT for MDS-RCC are scarce, with existing studies often focused on specific genetic subgroups or stem cell sources. This study aims to compare HCT outcomes for pediatric MDS-RCC and SAA and to identify clinical determinants, including exposure to anti-thymocyte globulin (ATG), associated with HCT outcome.
Methods
In this retrospective cohort study, consecutive patients with MDS-RCC or SAA without known genetic predisposition syndromes, diagnosed according to WHO/EWOG guidelines and treated between January 1994 and December 2021 in our institute, were included. Primary outcome was grade 2-4 acute graft-versus-host-disease (aGvHD)-free, chronic GvHD-free, event-free survival (GFEFS). Secondary outcomes included overall survival (OS), GvHD, and clinical determinants associated with these outcomes. These determinants included ATG exposure, calculated using a pharmacokinetic model. Univariable and multivariable analyses, taking along other known risk factors for HCT outcome (HLA match grade, stem cell source, CMV serostatus), were performed to determine risks.
Results
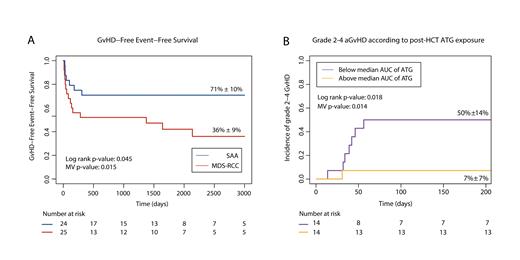
Forty-nine patients were included: 25 with MDS-RCC and 24 with SAA. The median age at HCT was 13.4 versus 10.2 years, respectively. Conditioning for MDS-RCC consisted mainly of treosulfan-based or busulfan-based regimens (56%and 36%, respectively); SAA patients were mainly conditioned with fludarabine-cyclophosphamide (63%) or cyclophosphamide only (29%). Bone marrow was used as a stem cell source in 82% of patients. At a median follow-up duration of 6.3 years, GFEFS of patients with MDS-RCC was 36%, compared to 71% in SAA (hazard ratio (HR) 0.44, p=0.079; Figure 1a). OS was 76% versus 96% (univariate p=0.14). The most frequent event to fail on GFEFS was grade 2-4 aGvHD, affecting 44% of patients with MDS-RCC and 8% of patients with SAA (HR 0.15, p=0.015). Grade 3-4 aGvHD was only observed in MDS-RCC. Corrected for dose and pharmacokinetics, ATG exposure was lower in patients with MDS-RCC compared to SAA (median post-HCT exposure 2.5 AU*day/L versus 31.9 AU*day/L, p=0.002). A similar trend was observed for pre-HCT ATG exposure 39.4 AU*day/L versus 61.3 AU*day/L, p=0.065). In multivariable analysis, low post-HCT ATG exposure was associated with higher incidence of grade 2-4 aGVHD (HR 0.90, p=0.014; Figure 1b).
Conclusion
HCT for MDS-RCC is associated with lower exposure to ATG and higher incidence of GvHD compared to SAA. Insight into the differential pharmacodynamics of ATG in children with acquired BMF is needed to improve HCT outcome.
Figure 1: Insufficient ATG exposure is associated with aGVHD after HCT for pediatric aBMF.
(a) Graft-versus-host-disease free, event free survival after HCT for pediatric severe aplastic anemia (SAA; blue line) or myelodysplastic syndrome-refractory cytopenia of childhood (MDS-RCC; red line). (B) Incidence of grade 2-4 acute GvHD in aBMF patients with post-HCT exposure to ATG below (purple) or above the median (yellow).
Disclosures
Nierkens:Sobi: Membership on an entity's Board of Directors or advisory committees. Lindemans:Sobi: Membership on an entity's Board of Directors or advisory committees; Orchard Therapeutics: Membership on an entity's Board of Directors or advisory committees; ExCellThera: Other: Data and safety monitoring board; Pfizer: Patents & Royalties: IL-22 in GvHD.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal